存在大肠杆菌细菌表明卫生问题
水系统中的微生物,特别是 致病细菌,威胁公共健康 [1]。人类和动物的废物可能导致 水源污染,其中大肠杆菌 (大肠杆菌)细菌 表明粪便污染,因为它们几乎只存在于人类 和动物的粪便中 [1]。
需要一个粪便污染的指示物来 保护公共健康 [2],而这正是大肠杆菌发挥作用的地方 [3]。大肠杆菌因此在 卫生指示中扮演着重要角色 [4],并被认为是确保公共健康的最佳生物饮用水 指示物 [2]。大肠杆菌菌株,特别是致病菌,指出水质和 卫生问题 [4] [5]。
因此,使用大肠杆菌污染作为指示物是一种经济有效的食品和水的检测方式,以保护 公共健康 [2],而无需 在单独的测试中检测一系列病原体。大肠杆菌可能表明其他食源性病原体的存在 [6],例如沙门氏菌, 李斯特菌, 志贺氏菌, 弧菌, 甲型肝炎 [7]。
致病性大肠杆菌O157通过金标准方法仍然未被检测到 O157 remain undetected by gold standard method
大肠杆菌是 革兰氏阴性、杆状细菌,栖息在 人类和动物的胃肠道中[8]。虽然大多数菌株是无害的, 但也有一些病原菌株可以根据 临床症状或血清群进行区分[8]。
表面上的脂多糖大肠杆菌细胞构成O抗原,决定O血清群,并且对宿主-病原体相互作用至关重要[9]。 例如,大肠杆菌O157:H7具有体表O抗原157和 鞭毛(H)抗原7[8]。这种特定菌株(大肠杆菌O157:H7)是 最严重的食源性病原体之一,导致严重疾病并因此 对公共健康构成重大威胁[8]。例如,在美国,大肠杆菌O157:H7最常通过 食用受污染的食物和水传播[8]。由于缺乏针对大肠杆菌O157:H7的特定治疗,预防和控制 变得更加重要[8]。
感染大肠杆菌O157可能变得非常危险,因为它 可能导致溶血性尿毒症综合症,这是美洲和欧洲儿童急性肾衰竭的最常见原因[10]。不幸的是,目前没有可用的治疗选项来 降低发展溶血性尿毒症综合症的风险[10]。
几乎所有大肠杆菌临床分离株产生β-D-葡萄糖醛酸酶, 这使得该酶在检测大肠杆菌在水、食品以及环境样本中,这些细菌表明可能存在粪便污染[11]。然而,我们在使用β-D-葡萄糖醛酸酶进行粪便检测时需要小心[11],因为大肠杆菌(E. coli)O157:H7无法产生β-葡萄糖醛酸酶[8]。 O157:H7 is not capable of producing beta-glucoronidase [8]. 因为大肠杆菌O157是β-葡萄糖醛酸酶阴性,因此不会被ISO平板法检测为大肠杆菌(E. coli)(ISO 9308-1:2014)。
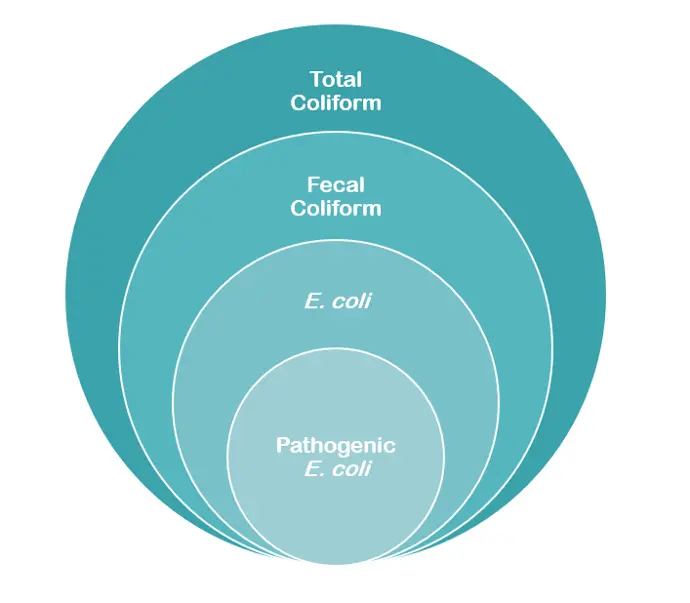
图表:从总大肠菌群到致病性大肠杆菌.
致病性、产志贺毒素的大肠杆菌(STEC)最常涉及食源性暴发,CDC估计STEC O157约占美国STEC感染的36%。然而,在培养皿上进行的金标准ISO方法未能检测到大肠杆菌O157。使用rqmicro方法的检测不依赖于酶,因此也能检测到特别危险的血清型大肠杆菌O157。 O157.
通过rqmicro的大肠杆菌检测工具,享受市场领先的结果时间和致病菌株的检测。
调查微生物饮用水质量需要检测指示性细菌,如大肠杆菌,这是饮用水中粪便污染的最佳细菌指示物[5]。检测和定量大肠杆菌在水中识别粪便污染并保持水的安全至关重要 [1]。
大肠杆菌O157:H7 负责许多疾病暴发,这就是为什么拥有快速可靠的检测方法如此重要 [12]。对污染的早期识别对食品行业至关重要 [13],并可能防止产品召回 [1]。检测方法需要高度敏感,因为大肠杆菌可能仅以非常低的水平存在 [1]。根据 ISO 9308-1:2014 的培养基检测方法未能检测到特别危险的 O157 型大肠杆菌,并需要 24 到 72 小时才能得出结果 [1]。尽管有更快的技术(PCR,ELISA),但它们成本高,需将样本运输到实验室,并涉及复杂的设备和程序,这些只能由经过培训的专业人员处理 [1]。[1]. Although faster techniques (PCR, ELISA) are available, they are expensive, require sample transport to the laboratory and involve complex equipment and procedures which can only be handled by trained professionals [1].
易于使用的rqmicroE.coli测试基于流式细胞术技术,包括特别危险的 O157 型大肠杆菌,并在同一班次内提供结果。根据需求,用户可以选择最低检测限(LOD)为 1 个细胞 / 100 毫升的定性结果,8 小时后可用,或在市场领先的 2 小时结果时间内,选择检测限(LOD)为 10 个细胞 / 100 毫升的定量结果。a market-leading time to result of 2 hours.
了解更多关于我们两种不同的大肠杆菌测试工具包的信息,请访问我们的 网站.
rqmicro.COUNT仪器的便携性使您能够直接访问食品和饮料行业的关键控制点。因此,rqmicro.COUNT将促进您的风险管理,使您能够确保水和食品产品的安全。
参考文献:
[1] Nurliyana, M. R.等人。"水资源中大肠杆菌的检测方法:综述。"《物理学杂志:会议系列》. 第995卷。IOP出版,2018年。
[2] Edberg, S. C. L.等人。"大肠杆菌:公共卫生保护的最佳生物饮用水指示物。"《应用微生物学杂志》88.S1(2000):106S-116S。
[3] Keshav, V.等人。"来自抹布的大肠杆菌作为家庭卫生状况的指示物。"《水、卫生和发展卫生杂志》5.3(2015):351-358。
[4] Scheinberg, Joshua A.等人。"从宾夕法尼亚州农贸市场获得的绿叶蔬菜、牛肉和猪肉中分离的大肠杆菌及卫生指示细菌的流行情况和系统发育特征。"《食品保护杂志》80.2(2017):237-244。
[5] Odonkor, Stephen T.和Joseph K. Ampofo。"大肠杆菌作为水的细菌质量指示物:概述。"《微生物研究》4.1(2013):e2。
[6] Truchado, Pilar 等. "大肠杆菌水平与表面灌溉水中食源性病原体存在之间的相关性:建立采样程序."水研究128 (2018): 226-233.
[7] Bintsis, Thomas. "食源性病原体."AIMS 微生物学3.3 (2017): 529.
[8] Lim, Ji Youn, Jang W. Yoon 和 Carolyn J. Hovde. "大肠杆菌 O157: H7 及其质粒 O157 的简要概述."微生物学与生物技术杂志20.1 (2010): 5.
[9] DebRoy, Chitrita, Elisabeth Roberts 和 Pina M. Fratamico. "大肠杆菌中的 O 抗原检测."动物健康研究评论12.2 (2011): 169-185.
[10] Pennington, Hugh. "大肠杆菌 O157."柳叶刀376.9750 (2010): 1428-1435.
[11] Chang, GEORGE W., J. A. N. I. N. E. Brill 和 R. O. S. A. L. I. N. D. Lum. "人类粪便样本中β-D-葡萄糖醛酸酶阴性大肠杆菌的比例."应用与环境微生物学55.2 (1989): 335-339.
[12] Deisingh, A. K. 和 M. Thompson. "检测食品中大肠杆菌 O157: H7 的策略."应用微生物学杂志96.3 (2004): 419-429.
[13] De Boer, E. 和 A. E. Heuvelink. "检测和分离产志贺毒素的大肠杆菌的方法."应用微生物学杂志88.S1 (2000): 133S-143S.